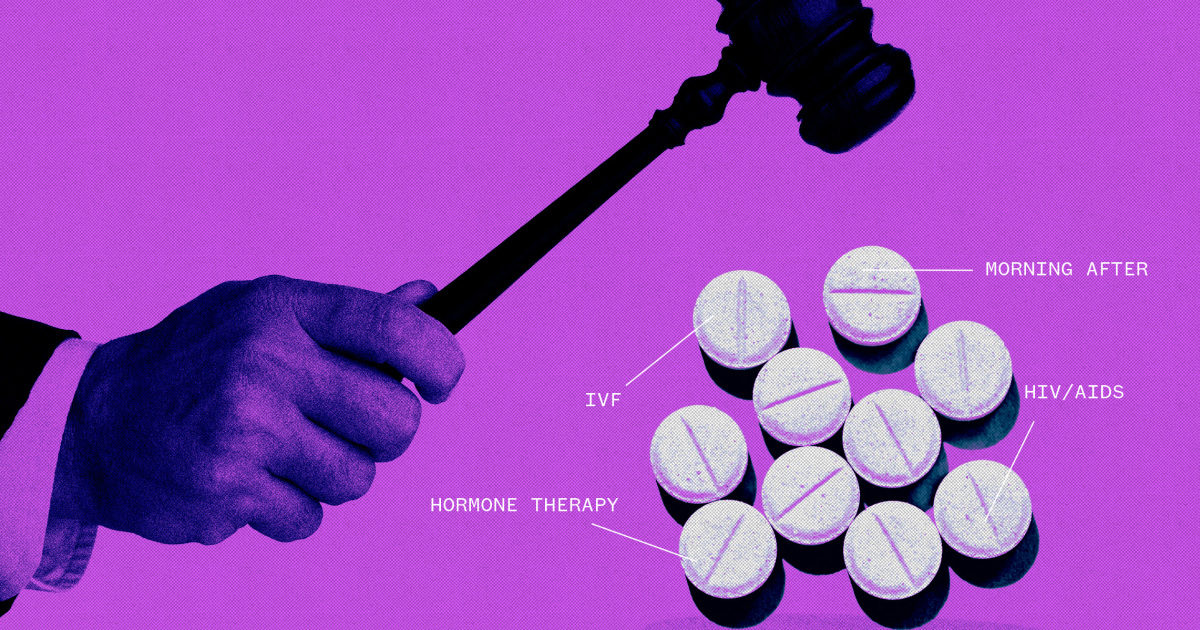
Vaccines, birth control pills, hormone therapies and fertility drugs would be subject to new litigation if the Supreme Court endorses a challenge to abortion pill mifepristone, pharmaceutical industry experts warn.
When the court on Tuesday weighs whether to roll back Food and Drug Administration findings that made mifepristone more readily available, it is not just access to that particular drug, used for the majority of abortions nationwide, that is on the line.
The pharmaceutical industry has raised the alarm, telling both the justices in court filings and anyone else who will listen that giving individual federal judges the power to cast aside the agency’s scientific health and safety findings would cause chaos within the sector.
It would likely lead to litigation over other drugs, both current and those yet to be approved, on which people have strong feelings.
If the anti-abortion groups win, “anyone with an ideological disagreement, coupled with a scientifically untrained judge, could challenge the FDA’s authority,” said Amanda Banks, a physician and entrepreneur who signed a briefalong with dozens of other pharmaceutical executives and companies backing the FDA.


This is the best summary I could come up with:
The pharmaceutical industry has raised the alarm, telling both the justices in court filings and anyone else who will listen that giving individual federal judges the power to cast aside the agency’s scientific health and safety findings would cause chaos within the sector.
For business leaders, there’s also a concern a ruling against the government could stifle innovation by deterring investors in an industry that relies on billions of dollars in upfront research and development in order to bring drugs to market.
“My biggest concern is the precedent it sets … could have a chilling effect on investors coming into our business and investing in our innovative companies,” said Paul Hastings, an industry veteran who is the CEO of Nkarta Therapeutics and signed on to the same brief as Banks.
When the agency in 2016 began the process of lifting restrictions on the drug, its actions were “supported by an exhausting review of a record including dozens of scientific studies and decades of safe use of mifepristone by millions of women,” the brief said.
The challengers, doctors and other medical professionals who oppose abortion argue that FDA failed to sufficiently take into account safety concerns when the restrictions on mifepristone were lifted.
The plaintiffs sued in a federal court in Texas where the case was guaranteed to be assigned to Matthew Kacsmaryk, a conservative judge appointed by President Donald Trump.
The original article contains 1,131 words, the summary contains 230 words. Saved 80%. I’m a bot and I’m open source!